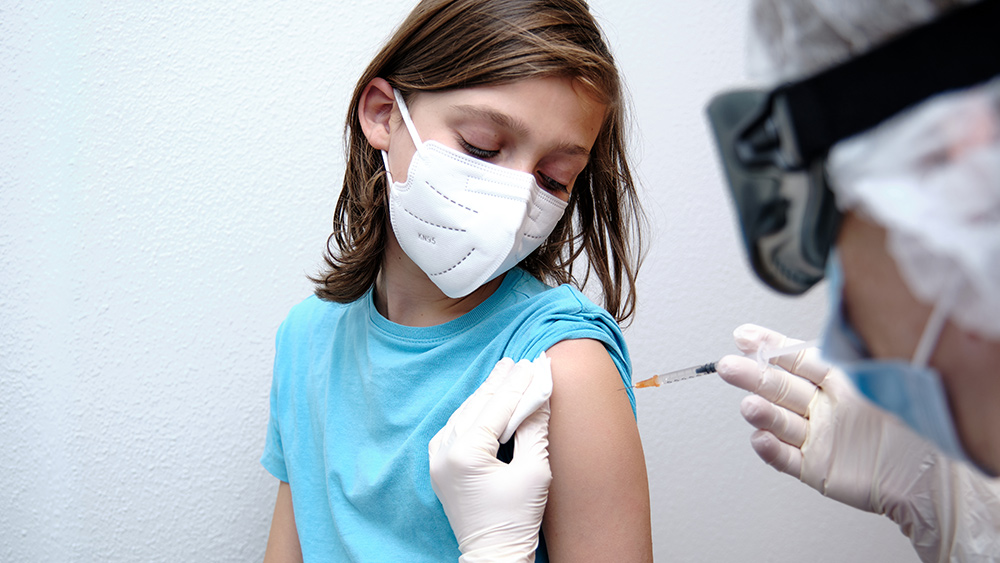
The FDA recently expanded its Emergency Use Authorization (EUA) for the monkeypox vaccine, allowing it to be used in children under 18 who are considered to have a “high risk” of infection.
Before the updated EUA, “numerous” children had been given the vaccine via a special permission process despite not being authorized or approved for children, the FDA confirmed. The median age of people being infected by the virus is 35, and very few cases involving children have been reported so far in the U.S.
Are there really that many children at risk of contracting a disease that is primarily spread through male homosexual activity? Some have made the argument that some children are exposed to the disease because they are being abused, but wouldn’t a better solution be to remove these children from the abusive situations that are putting them at risk?
According to the CDC, children who have a higher risk for severe monkeypox are those who have an abnormal infection involving their face, genitals or eyes; are under eight years of age; have a history of skin conditions such as psoriasis or eczema; or have an immunocompromising disease. Is every child who got a COVID-19 vaccine now at a higher risk of monkeypox and many other illnesses thanks to its weakening effect on their immune systems?
The EUA also expanded the vaccine options for the disease to include administering the Jynneos smallpox vaccine via intradermal injection. This means it can be injected between the layers of the skin instead of underneath it. This would increase the number of available doses fivefold as the method only uses a fraction of the dose while allegedly offering the same protection. The current 441,000 available doses could be stretched to 2.2 million using the approach.
New intradermal injection strategy called into question
However, guidance issued by the CDC “discourages variations from the recommended route, site, volume or number of doses of any vaccine” on the grounds that “variation from the recommended route and site can result in inadequate protection.”
In fact, the CDC’s own website lists several examples of cases where the method with which a vaccine is administered can alter its immunogenicity, or its ability to provoke an immune response.
Some experts have expressed concerns about the low-dose intradermal strategy. The Executive Director of the National Coalition of STD Directors, David Harvey, said in a statement: "This approach raises red flag after red flag, and appears to be rushed ahead without data on efficacy, safety, or alternative dosing strategies."
Those under 18 must get the vaccine via subcutaneous injection, which goes below the skin. The shots are administered in a series of two doses spaced 28 days apart.
The FDA’s new authorization came less than a week after monkeypox was declared a public health emergency by the Biden administration. As many people learned during the Covid pandemic, this gives government health agencies the power to authorize the use of medical products that have not been approved, along with permission to use approved medical products in a different way than intended to stop a virus from spreading.
Monkeypox is a disease that is rarely fatal, and so far, no deaths in the U.S. have been attributed to it. It is highly treatable, although it is unpleasant to experience and some patients must be hospitalized to manage their painful lesions.
There is one fact that really stands out about monkeypox: 98 percent of monkeypox patients in the U.S. who shared their demographic information were men who have sex with men. Therefore, pushing this jab on children is coming across as yet another Big Pharma money grab, especially when being immunocompromised is enough to put someone in the “high risk” category just as so many people are dealing with weakened immune systems thanks to Covid vaccines.
Sources for this article include:
Please contact us for more information.


















