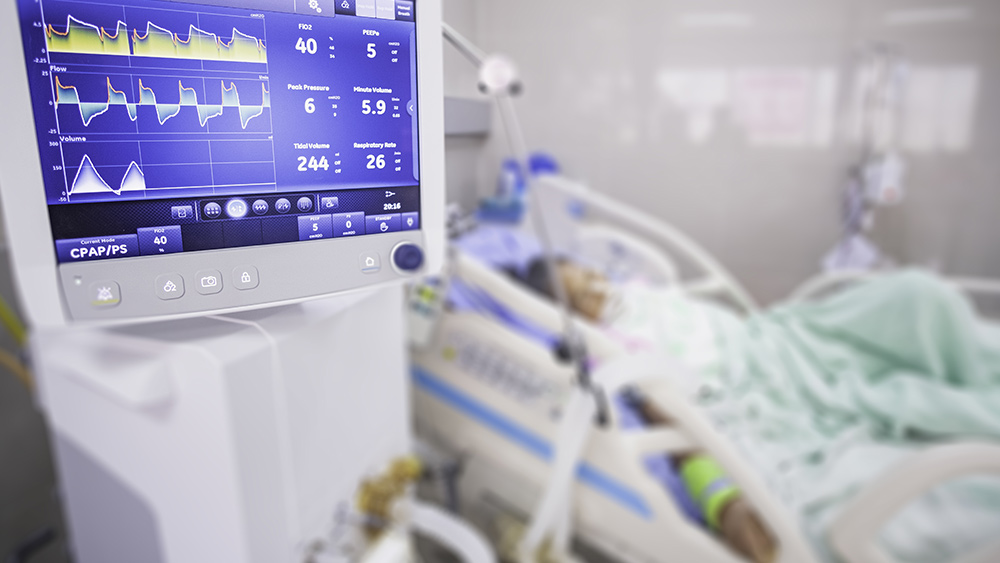
The Centers for Disease Control and Prevention (CDC) on Tuesday, January 4, cut the recommended interval between initial inoculation and boosting from six to five months for Americans who are fully vaccinated with Pfizer's Wuhan coronavirus (COVID-19) vaccine.
Booster interval recommendations for those who got Johnson & Johnson or Moderna remain at two and six months, respectively.
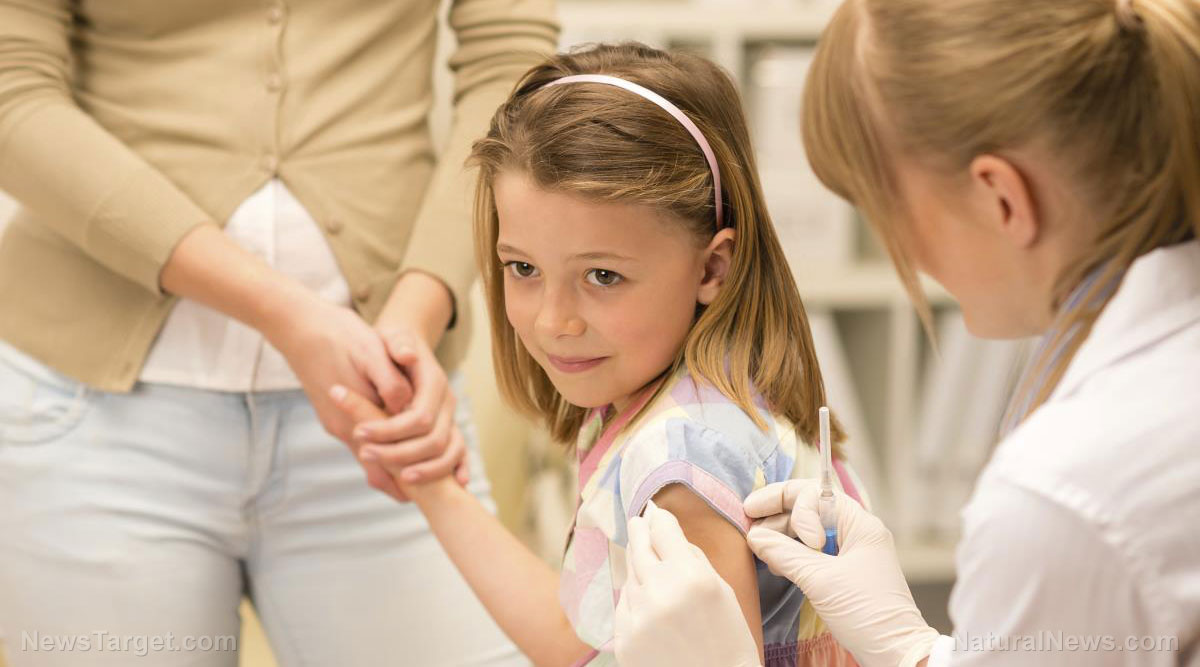
The agency, which is now being taken to court for refusing to publicize the post-licensure COVID vax safety data, is also advising moderately or severely immunocompromised children between ages five and 11 to get an additional dose of the jab 28 days after their second shot. At present, only the Pfizer vaccine is accredited and endorsed for children of the said age. (Related: Fauci floats the idea of injecting eligible Americans with COVID booster shots every six months.)
CDC Director Rochelle Walenski, who announced the changes, reassured the public that her agency will continue to update their recommendations to ensure the best possible protection for the American people while persuading all eligible Americans to receive a booster for themselves and their vulnerable children.
FDA approves boosters for 12 to 15 years old
Walenski also notified Americans that the Food and Drug Administration (FDA) has approved boosters for children 12-15 years old earlier this week.
This is the same FDA that is aware of the tens of thousands of people who suffered severe side effects and more than a thousand deaths that happened in vaccinated people within the first two and a half months of the Pfizer vaccine rollout. The agency is now being sued for refusing to disclose the Pfizer safety data it used to license the shot.
Pfizer CEO Albert Bourla, meanwhile, was contented with the developments made by the FDA on shortening the waiting period between the second and third doses. Bourla said that his company continues to believe that broad use of boosters is essential to preserving a high level of protection against COVID-19, reducing the rate of hospitalizations and helping defeat the pandemic.
Over a month ago, Bourla stated that after people get triple-vaccinated, they will have to be "re-vaccinated" against COVID on a yearly basis. He said that the virus continually mutates and the immunity given by the jabs wanes over time.
The move to cut the Pfizer booster interval came as the United States reported more than a million new cases of COVID-19 on Monday, Jan. 3, a new record that far exceeds the worst days of last winter's surge. However, experts say that the case record is likely to be an undercount given the current availability of at-home tests.
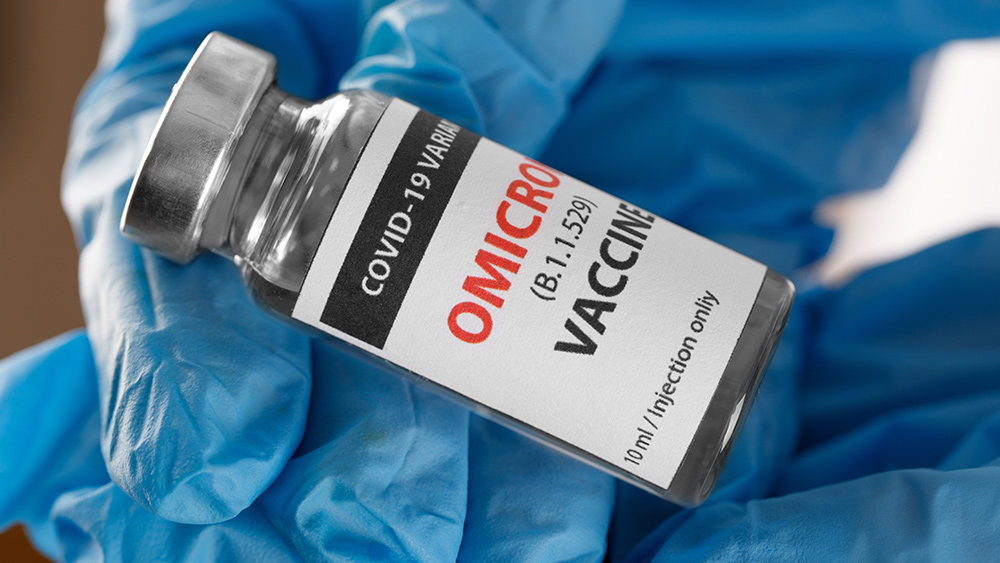
The CDC presently estimates that more than 95 percent of COVID-19 infections in the U.S. are caused by the omicron variant, which is more transmissible than the earlier variants – especially among those who are vaccinated.
Spike proteins from COVID vaccines become targets of autoimmune attack
Cardiologist Dr. Peter McCullough explained that due to the extensive memory-type antibody response to SARS-CoV-2, the antibodies from COVID vaccines can lead to the destruction of any cell that manufactures the virus' spike protein. Additional doses will make the spike proteins grow further and become targets of an autoimmune attack.
Dr. Sucharit Bhakdi, who advised people to give up any additional doses of the vaccines to cut the risk of autoimmune damage, detailed the same mechanism along with Dr. Arne Burkhardt. Bhakdi found that 14 of the 15 vaccinated patients who died suffered an autoimmune damage in various organs.
Meanwhile, Sir Andrew Pollard, the director of the Oxford Vaccine Group and chair of the United Kingdom’s Committee on Vaccination and Immunization, declared his opposition to the booster doses for the general population.
"We know that people have strong antibodies for a few months after their third vaccination, but more data is needed to assess whether, when, and how often those who are vulnerable will need additional doses," Pollard stated. "We can’t vaccinate the planet every four to six months. It's not sustainable or affordable. In the future, we need to target the vulnerable."
He added that the combination of natural immunity and the immunity gained from the initial doses of the vaccines are strong enough to protect against the omicron variant, which is much less severe compared to the delta variant.
Official bodies like the World Health Organization and the European Commission have warned against the use of COVID booster shots whose safety and efficacy have not been studied. In the U.S., all of the presently available COVID jabs for adults have yet to complete clinical trials with the earliest completion date slated on May 2023.
Watch the video below to learn the truth about COVID-19 vaccines.
This video is from the Anti-Disinformation channel on Brighteon.com.
Follow Vaccines.News for more news related to COVID-19 vaccines.
Sources include:
Please contact us for more information.