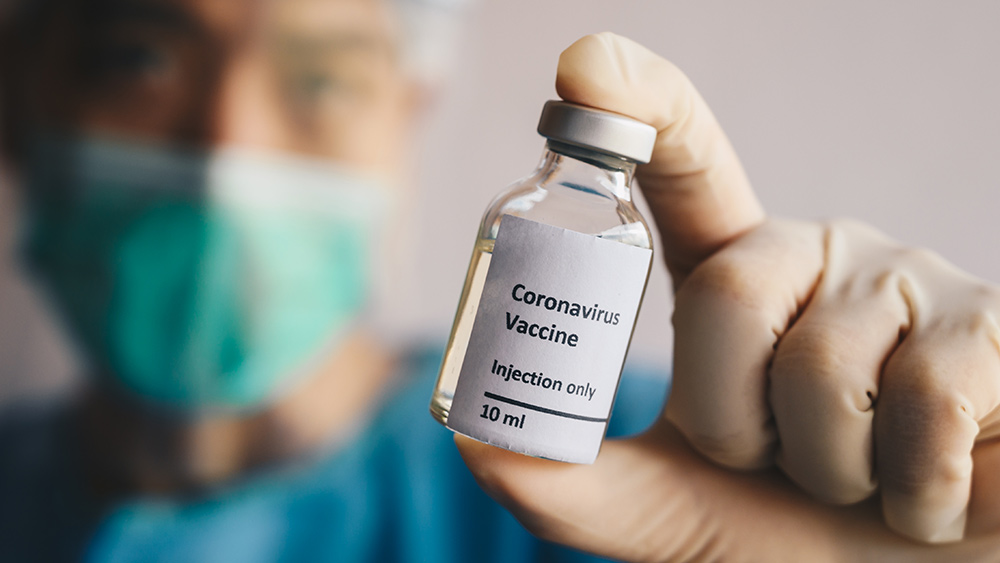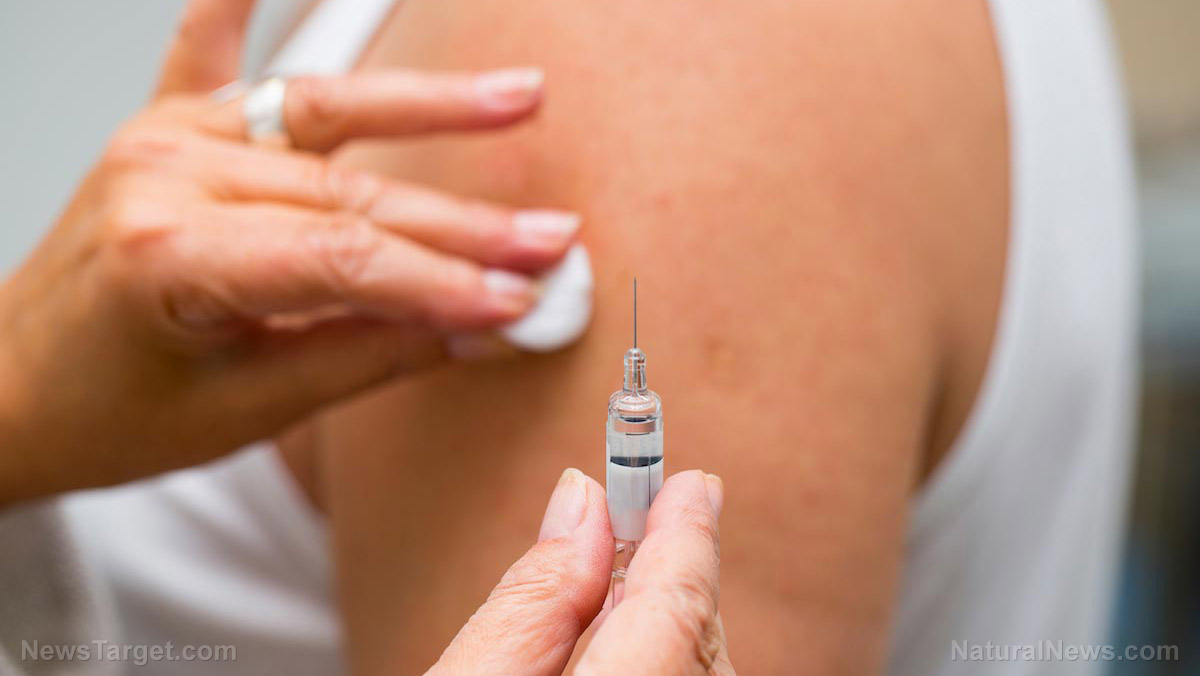
China has promised to fast-track the development of an effective coronavirus vaccine before the year ends and share this vaccine with other countries as part of its contribution to the fight against COVID-19. However, some have questioned China’s capability to produce sufficient doses. They also raised concerns with the country's deals with individual nations.
Chinese leader Xi Jinping told the World Health Assembly in May that his country’s coronavirus vaccines would be a “global public good.” He and Chinese premier Li Keqiang have publicly pledged to make Chinese coronavirus vaccines available to different countries in Southeast Asia, Africa and Latin America.
Zheng Zhongwei, the director of the Chinese National Health Commission’s Development Center for Medical Science and Technology, said in September that the country could produce 600 million doses by the end of 2020 and more than one billion in 2021.
In October, China announced it had joined COVID-19 Vaccine Global Access (COVAX) – a collaborative effort by Gavi, the Vaccine Alliance, Coalition for Epidemic Preparedness Innovations (CEPI) and the World Health Organization (WHO). COVAX aimed to provide 2 billion vaccine doses to health workers and the most vulnerable, especially in developing countries.
Concerns raised over the safety of vaccines and China’s manufacturing capability
However, these commitments would leave insufficient amounts of doses available for export – given that majority of China’s 1.4 billion population has not been vaccinated. Klaus Stöhr, former head of the WHO’s epidemic response unit, said: “The number of doses available in China will be too little to permit export unless a political decision is taken to ship vaccines overseas despite still-existing vaccine needs.”
International Vaccine Institute Director-General Jerome Kim expressed worry that China might use its COVID-19 vaccine deals with individual countries for political or economic leverage in the future, something he called “regrettable.”
In addition, scientists have said that Chinese vaccines should first be firmly established as safe and effective before given to more people. They urged China's drug regulator – part of the Ministry of Health – to wait for trial data proving the vaccines' safety and effectiveness before approving them.
Wu Guizhen, chief biosafety officer at the Chinese Center for Disease Control and Prevention, said in an email to the Nature journal that the health ministry will await the results of large trials before approving any vaccine for sale. “Until then, there are still uncertainties,” she added.
Wu also said in the email: “It is also necessary to obtain the valid data of phase III clinical trials to fully assess the safety and effectiveness of the vaccines.”
A number of countries look forward to China’s coronavirus vaccines despite concerns
Crucial phase three trials for four vaccine candidates developed by Chinese pharmaceutical companies are still in progress as of writing. Shanghai-based Sinopharm developed two COVID-19 jabs, while the other two came from Beijing-based Sinovac and CanSino Biologics in Tianjin. (Related: INHALE the COVID: Beijing has approved a nasal spray vaccine for clinical trials.)
Of the four coronavirus vaccine candidates, only those from Sinopharm and Sinovac have been used in other countries under emergency-use authorization.
Sinopharm's vaccines are being tested in several countries, including the United Arab Emirates, Bahrain, Peru and Argentina – the last nation seeing widespread media coverage of large-scale vaccine trials. Laboratorio Elea Phoenix Scientific Director Eduardo Spritzer, whose facility is organizing the vaccine tests, said: “We are working as fast as possible, but without losing quality in the data obtained from the trials.”
Meanwhile, Sinovac had struck a deal with the state of Sao Paulo in late September for 60 million coronavirus vaccine doses costing US$90 million. An Oct. 3 report by Reuters said Sao Paulo governor Joao Doria had asked approval from Anvisa, the Brazilian health regulator, to use Sinovac’s coronavirus shots on the country’s most populated state.
The WHO and CEPI need to approve any Chinese vaccine if the country commits to giving doses; so far, none of these candidates are on CEPI’s list of vaccines it supports. China will also have to increase its manufacturing capacity if it is to provide vaccines.
Just like Operation Warp Speed in the U.S., China’s attempt to fast-track the vaccine's development to protect against the coronavirus should not dismiss potential safety concerns.
Sources include:
Please contact us for more information.