Moderna Chairman Noubar Afeyan admitted that the company's Wuhan coronavirus (COVID-19) vaccine may require yearly boosters. "It may well need an annual booster, potentially varying on a year-to-year or every few years basis as the virus varies," he said in an interview with Maria Bartiromo.
Afeyan noted that the impact and the death toll of the pandemic have been unlike anything the world had seen in the past century. Thus, there is a lot of uncertainty surrounding what the virus could look like in the future.
"We just don't know how this virus is going to travel from being a pandemic all the way to potentially an endemic virus we have to get used to living with. I think if we end up there, there will be a continuous need for boosting," he said.
Moderna developing vaccine that combines COVID-19 booster and flu shot
In September, Moderna said it is developing a single vaccine that combines the COVID-19 booster dose with an experimental flu shot. Afeyan told Bartiromo that the company hopes to have data regarding the seasonal flu vaccine soon. "We are hopeful that we will see similarly encouraging results from the whole mRNA platform so that we are able to show robust and effective vaccination." he said.
Moderna is also looking to develop more vaccine combinations so that people can think of vaccines as a type of protection and "as a part of how we’re living, coexisting with viruses that are increasing in terms of the threat to us."
The Centers for Disease Control and Prevention (CDC) recently approved mixing and matching boosters, so people who are eligible for additional shots can decide which brand of vaccine they can get, as all three vaccines authorized by the Food and Drug Administration (FDA) are considered to be "extraordinarily safe" and "effective."
People who received their vaccinations months ago are now eligible for a booster. With different shots available as boosters, over 9,800 CVS pharmacies across the nation are now offering the Moderna booster to eligible populations, along with the Pfizer-BioNTech COVID-19 booster.
Consumers who are six months past their last vaccination are urged to get boosters if they are 65 or older, nursing home residents or are at least 50 and have an increased risk of severe disease due to health problems.
Adults of any age who are at increased risk of infection due to health problems, their jobs or living conditions are also encouraged to get boosters. These include health care workers, teachers and those in jail or are in homeless shelters.
Billions at stake for vaccine manufacturers
With the number of doses rolling out, including booster shots, it begs to question what vaccine manufacturers have to gain this pandemic – or how much. Billions more in profits are at stake for manufacturers like Moderna, Pfizer, and Johnson & Johnson as the country moves forward to dispensing booster shots to increase "protection" against the virus.
The average forecast for Moderna's 2022 revenue has already jumped 35 percent since President Joe Biden laid out booster plans in mid-August. While nobody knows how many people will get the extra shots yet, analysts expect boosters alone to bring in over $14 billion in sales for Moderna.
For Pfizer and Moderna, booster shots could be more profitable because they would no longer come with the research and development costs that they previously incurred to get the initial vaccines in the market.
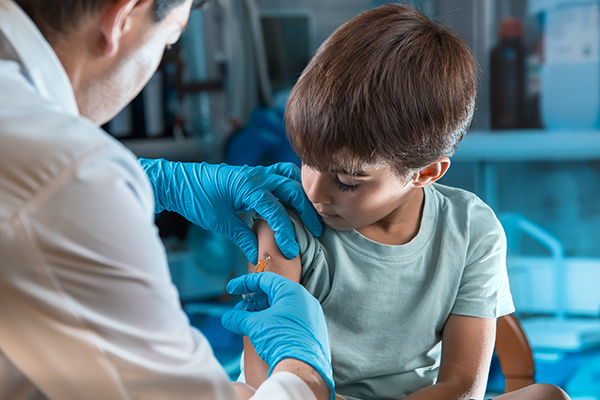
After the adult doses, Moderna is moving toward vaccinating children. Afeyan said that the low dose of Moderna's COVID-19 vaccine is safe and effective for children aged 6 to 11 and that the company will join its rivals in moving toward expanding shots for children. (Related: Most parents reject COVID-19 jabs for children aged 5 to 11 - poll.)
Moderna is still waiting to be greenlighted to offer its vaccine to teens but is already studying lower doses in younger children. Pfizer, on the other hand, has vaccine doses for children nearing widespread use after getting emergency use authorization from the FDA.
Get more updates about COVID-19 vaccine development at Pandemic.news.
Sources include:
Please contact us for more information.