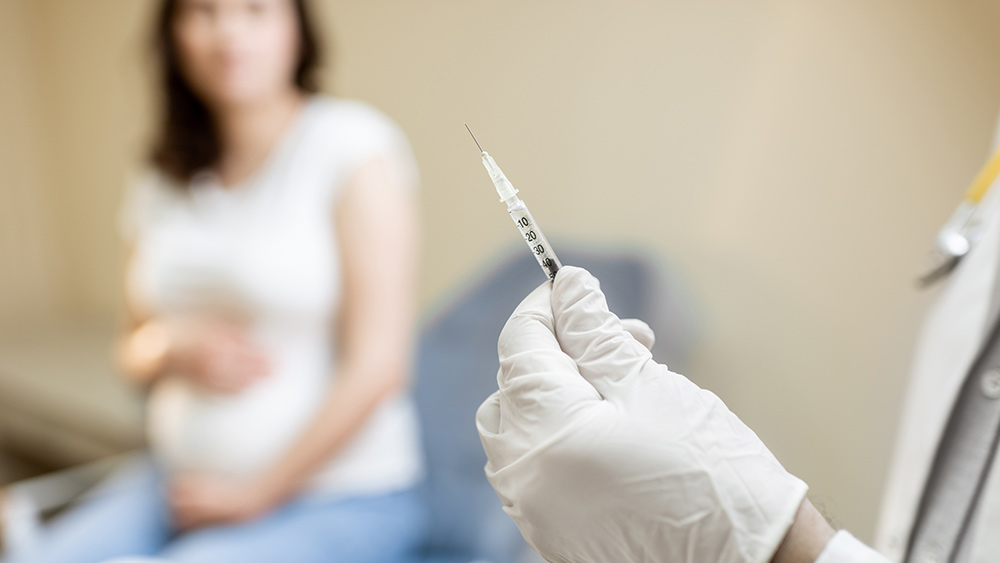
Brazil paused the use of AstraZeneca's coronavirus (COVID-19) vaccine on pregnant women following guidance from its federal health regulator. The "immediate suspension" order for the vaccine issued on May 11 stemmed from the death of a pregnant woman after inoculation. Two Brazilian states earlier halted the vaccine's use for pregnant females.
Brazil's National Health Surveillance Agency (Anvisa) recommended that the AstraZeneca vaccine be suspended immediately in a May 10 statement. According to the statement, Anvisa issued the guidance after "constant monitoring of adverse event related to [COVID-19] vaccines in use in the country."
The state of Sao Paulo previously suspended the vaccine for pregnant women with previous medical conditions. On the other hand, Rio de Janeiro state suspended it for all pregnant women. Both states cited Anvisa's guidance as a factor in their pause of the AstraZeneca COVID-19 vaccine.
Rio de Janeiro Health Secretary Alexandre Chieppe confirmed the pregnant woman's death in the state. He said the 35-year-old woman, who was 23 weeks pregnant, died after getting immunized with the AstraZeneca vaccine. She died of a hemorrhagic stroke on May 10, five days after checking into a hospital.
It is unclear if the Rio de Janeiro woman had any underlying diseases. Anvisa however said that her demise "was assessed as possibly related to the use of the vaccine." Health authorities are now investigating the woman's death.
Meanwhile, the British drug manufacturer said in a statement to Reuters that its vaccine clinical trials did not include pregnant and lactating women. It added that studies in animals did not produce direct or indirect evidence of harm on pregnancy and fetal development.
The British company partnered with the Brazilian public health institute Oswaldo Cruz Foundation (Fiocruz) to distribute its vaccine in the country. It is one of three COVID-19 vaccine candidates permitted in the largest South American nation. Brazil also approved the vaccines manufactured by Sinovac Biotech and Pfizer for use there.
AstraZeneca's vaccine has always been problematic
The AstraZeneca vaccine had already caused the death of another Brazilian – seven months before the pregnant woman died. Back in October 2020, The Epoch Times reported that a volunteer who joined clinical trials there died. Anvisa confirmed the Brazilian volunteer's death but remarked that it would not halt vaccine trials.
Later, two health workers in Denmark suffered brain hemorrhages after they were inoculated with the AstraZeneca vaccine. The two Danes experienced adverse reactions less than 14 days after their vaccination. One of the two patients eventually died. The Danish Medicines Agency confirmed the two cases, but did not elaborate on the matter. (Related: Danish regulator says woman who died after AstraZeneca jab had "unusual symptoms" including from blood clots.)
The Danish Health Authority (SST) initially ordered a temporary suspension of the vaccine in March alongside more than 20 other European nations that used it. Most of these countries eventually resumed use of AstraZeneca's vaccine. However, Denmark made the suspension on the AstraZeneca permanent. (Related: Denmark permanently stops rollout for AstraZeneca vaccine, citing concerns about blood clots.)
SST Director General Søren Brostrøm said in an April 14 statement: "Based on the scientific findings, our overall assessment is there is a real risk of severe side effects associated with using the COVID-19 vaccine from AstraZeneca. We have, therefore, decided to remove the vaccine from our vaccination program." He remarked that the move to permanently suspend the AstraZeneca vaccine "has been a difficult decision." Brostrøm nevertheless commented that Denmark has other vaccines at its disposal.
Meanwhile, Denmark's neighbor Norway also recommended suspending the use of the AstraZeneca vaccine for its COVID-19 immunization program. Norwegian Institute of Public Health Infection Control and Environmental Health Division Director Geir Bukholm said on April 15: "We now know significantly more about the connection between the AstraZeneca vaccine and the rare and serious incidents of low platelets, blood clots and bleeding. Based on this knowledge, we have arrived at a recommendation that the AstraZeneca vaccine be removed from the coronary vaccination program in Norway."
Bukholm's statement followed an investigation performed by Oslo University Hospital researchers. The team led by Chief Physician Dr. Pål André Holme looked at cases of blood clots in Norway that followed the AstraZeneca vaccination. All of the reactions there occurred in healthcare workers below 50 years old.
Speaking to Norwegian newspaper VG, Holme said he is confident that his team identified antibodies responsible for the blood clots – which the AstraZeneca vaccine triggered. "Our theory is that this is a strong immune response that most likely comes after the vaccine. There is no other thing … that can explain this immune response. I'm pretty sure it's the antibodies [in the vaccine] that's the cause," he commented.
Visit VaccineDeaths.com to read more articles about the fatalities associated with the AstraZeneca Wuhan coronavirus vaccine.
Sources include:
Please contact us for more information.